WHAT I THOUGHT I KNEW
WHAT I THOUGHT I KNEW Wednesday, 22 July 2009
Oprah’s 5th Favourite Book
 WHAT I THOUGHT I KNEW
WHAT I THOUGHT I KNEW WHEN DES IS TAKEN OFF THE MENU

“The TGA has advised the Department that DES is not a therapeutic good currently on the Australian Register of Therapeutic Goods and is not currently available via any avenue of supply in Australia. As such, it is not the role of the TGA to provide information about DES or to promote public health messages to DES exposed women”.
[Correspondence 16/2/09 by Senator the Hon Jan McLucas, Parliamentary Secretary to the Minister for Health and Ageing]
The situation described above is dire, not only for those affected by DES exposure, but for Australians affected by any other harmful drug on the market which might be withdrawn from the Australian Register of Therapeutic Goods. The absurdity is that this policy allows a drug, dangerous enough to have important warnings issued, to then be deemed not dangerous enough to continue warning those affected after the drug is made unavailable.
The risk in this policy is that following the drug’s cancellation from the Register, the Department of Health and Ageing may decide not to issue further vital health information to the unfortunate victims of the drug. This is precisely the case for DES exposed Australians where the Department holds the opinion that promoting information about DES creates community anxiety. DES was on the Register for usage in prostate cancer until 1992 when it was cancelled.
Added note 27/7/09:
This month, DES Action Australia-NSW wrote to the Australian Chief Medical Officer requesting his personal assistance in rectifying this matter, so that accountability in promoting public health messages about DES is maintained similarly as before. In reply (23 July 09) we are assured the Department and the TGA are working together to ensure that updated information will continue to be available to DES women. This is unconvincing whilst ever the Department and TGA continue in policy/opinion not to promote health messages about DES directly to the public. Also, without one section of the Department wholly accountable for this task, there is real risk of the responsibility for this extremely serious health issue being transferred across sections of the Department.
To the complete detriment of DES exposed Australians, the Department continues to ignore the fact that many people remain unaware of their exposure and the potential adverse health effects of DES. By only placing information about DES on Department websites, this is simply not enough when many people remain unaware of the health dangers of DES.
DES Action Australia-NSW will continue lobbying on this matter and urges anyone affected to subscribe to the organisation to receive firsthand any vital health information about DES.
Friday, 29 May 2009
DES EXAMINATION EXPLAINED
 The organisation, DES Action Australia-NSW is often asked about this procedure. Here is some information for you.
The organisation, DES Action Australia-NSW is often asked about this procedure. Here is some information for you.Although the incidence of the rare cancer, clear cell adenocarcinoma of the cervix/vagina is small in DES daughters (about 1:1,000), the “DES examination” is recommended every year throughout their lives. The reasons are: the usual Pap test may not detect this cancer, this cancer type is aggressive and should be detected early, and there is no known upper age limit for this cancer’s occurrence. To get the best treatment, see a specialist experienced in the care of DES exposed people. (Specialist listing at blog article 11/2/09)
The DES examination is similar to the Pap test procedure, but the differences are important. It involves:
- A careful visual inspection and palpation (feeling) of the entire vagina.
- Separate Pap smears from around the outside of the cervix and from the entire upper vaginal walls (4-quadrant smears).
- An internal pelvic examination.
This examination may also include iodine staining of the vagina and cervix (normal tissue stains brown). Depending on the results of these tests, further procedures may be necessary, such as colposcopy and biopsy. At your first DES examination, your specialist may consider a colposcopy to be a good idea in providing a baseline for future management.
NOTE: A colposcopy is done with a colposcope, a device that works like a magnifying glass. It is placed on a stand between you and the doctor and does not enter your body. A biopsy is when a small piece of tissue is removed for study under a microscope.
DES daughters who have annual DES examinations can safely forgo the recommended two yearly Pap test as for the general population. In 2008, a US study of 3,140 DES exposed women showed, astonishingly, that a third of these women are not receiving their recommended annual DES examinations.
More information is at www.desaction.org/paptest.htm
Tuesday, 12 May 2009
DES BRANDS GALORE
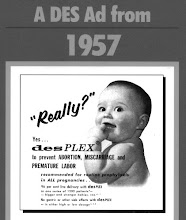
 Never patented, the synthetic oestrogen DES was marketed by many pharmaceutical companies. In fact, there were over 200 brand names for the drug. It was usually given in tablet form, but sometimes as injection, vaginal suppositories or combined in vitamin supplements.
Never patented, the synthetic oestrogen DES was marketed by many pharmaceutical companies. In fact, there were over 200 brand names for the drug. It was usually given in tablet form, but sometimes as injection, vaginal suppositories or combined in vitamin supplements.
It should be noted that DES was also used to stop breast-feeding after giving birth and this is not known to cause any problems. In the latter years of DES usage, another hormone called progesterone was more commonly used in pregnancy, but this hormone is not known to cause any problems.
In 2006, DES Action Australia-NSW sought a listing of Australian DES brands from Melbourne’s Royal Women’s Hospital Pharmacy Department. This list, although not exhaustive, may be useful for women who can recall being given some type of medication during pregnancy in the period of DES usage: 1938-1971 (and sometimes beyond). We are pleased to have received the following information:
The list below is some of the brands and generic names of products available in Australia and is not exhaustive, as there is no complete listing (ie, a list which contains all the generic and proprietary drug names that were available during the period 1940-1972).
List 1 - Products containing stilboestrol and hexoestrol
These drugs are more likely to have been prescribed for habitual abortion, threatened abortion, prevention of toxaemia, or diabetes in pregnancy and they have been linked to cervical or vaginal changes of the 'DES syndrome'.
Brand name (Generic name in italics)
Antigerant Hexoestrol
Clinestrol Stilboestrol
Cryogene B Stilboestrol Dipropionate
Cyrogene A Stilboestrol
Diesavite Stilboestrol
Euvalerol M Stilboestrol
Honvan Stilboestrol Diphosphate
Menotone Stilboestrol
Neo-oestranol Stilboestrol
Oestrogenine Hexoestrol
Pabestrol Stilboestrol
Pabestrol D Stilboestrol Dipropionate
Synthovo Hexoestrol
Thyboestrol Hexoestrol
List 2 - Products containing oestrogenic compounds
These products were available during the period in question but are less likely to have been used for pregnancy related problems and possibly be linked to 'DES syndrome'.
Brand name (Generic name in italics)
Ambigen Ethinyloestradiol & Methyltestosterone
Amenorone Ethinyloestradiol & Ethisterone
Amenorone Forte Ethinyloestradiol & Ethisterone
Barboestrol Ethinyloestradiol, Phenobarb & Papervine
Climatost Ethinyloestradiol, Methyltestosterone & Amphetamine Sulphate
Dienobarb Dienoestrol
Disecron Oestrodiol Monobenzoate & Progesterone
Duogynon injections Oestradiol Benzoate & Progesterone
Duogynon oral Ethinylosetradiol & Noresthisterone
Enavid Ethinyloesrtadiol 3-Methyl Ether & Norethynodrel
Estigyn Ethinyloestradiol
Estigyn Elixir Ethinyloestradiol
Estinyl Ethinyloestradiol
Ethidol Ethinyloestradiol
Eticyclin Ethinyloestradiol
Femandren Ethinylostradiol & Methyltestosterone
Lut-Ovocyclin Oestradiol Dipropionate & Progesterone
Menoform injections Oestrone
Menoclimax Dienoestrol
Menstrogen injections Ethinyloestradiol & Progesterone
Menstrogen tablets Ethinyloestradiol & Ethisterone
Mepilin Ethinyloestradiol & Methyltestosterone
Mepilin Elixir Ethinyloestradiol & Methyltestosterone
Mestrone Ethinyloestradiol & Methyltestosterone
Mixogen Ethinyloestradiol & Methyltestosterone
Neo-oestrogenine* Dienoestrol
O.C.P** Dienoestrol
Oestroform Preparations of "Pure natural Oestrogenic Hormones"
Oestroform Aqueous Oestradiol Monobenzoate Suspension
Oestrogenine compound** Dienoestrol
Oramen Ethinyloestradiol & Ethisterone
Orasecron Ethinyloestradiol & Ethisterone
Ovestin Oestradiol
Ovocyclin Oestradiol Dipropionate
Pausandryl Ethinyloestradiol & Methylandrostanolone
Premarin Conjugated Oestrogens (Equine)
Premarin with Meprobamate Conjugated Oestrogens & Meprobamate
Premarin with Methyltestosterone Conjugated Oestrogens & Methyltestosterone
Primodian Ethinyloestradiol & Methyltestosterone
Primodian Depot Oestradiol Valerate & Testosterone
Primogyn C Ethinyloestradiol
Primogyn Depot Oestradiol Valerianate
TACE Chlorotrianisene
Theelin (capsules, ointment, pessaries) Oestrone
Vallestril Methallenoestrol
Viraxasterol Dienoestrol
* Products more likely to have been used for problems in pregnancy
**In 1963 the formulation of these products were altered. From that time, the hormonal component has been ethinyloestradiol
Non-Australian DES type products list is also available at the American CDC website for consumers:
http://www.cdc.gov/des/consumers/about/history.html (JAMA 236(10): 1107-1109, Sept 1976).
Monday, 27 April 2009
A REAL MULTICULTURAL AFFAIR
 There would be people residing in Australia who were DES exposed in overseas countries and in some of these countries, DES usage in pregnancy extended well beyond 1971. We also have reports of DES usage in pregnancy in Australia in the mid 1980s. Here is a listing of some countries involved: USA, Canada, Ireland, France (usage peaked 1976), UK, Netherlands, New Zealand, Belgium, Czechoslovakia, Finland, Germany, Italy, Norway, Portugal, Spain and Switzerland. Lately, our organisation has heard of cases in Philippines, Israel, Mexico, New Guinea, Malaysia, Kenya, Namibia, Nairobi, India (reported usage 1975), Indonesia, Egypt, Russia, Germany (1973) and Poland (as late as 1991).
There would be people residing in Australia who were DES exposed in overseas countries and in some of these countries, DES usage in pregnancy extended well beyond 1971. We also have reports of DES usage in pregnancy in Australia in the mid 1980s. Here is a listing of some countries involved: USA, Canada, Ireland, France (usage peaked 1976), UK, Netherlands, New Zealand, Belgium, Czechoslovakia, Finland, Germany, Italy, Norway, Portugal, Spain and Switzerland. Lately, our organisation has heard of cases in Philippines, Israel, Mexico, New Guinea, Malaysia, Kenya, Namibia, Nairobi, India (reported usage 1975), Indonesia, Egypt, Russia, Germany (1973) and Poland (as late as 1991).At a 1992 symposium held in Sweden [J. Psychosom. Obstet. Gynaecol. 14 (1993) 71-89], concerns were raised that DES might still be given to pregnant women in many parts of the world, including East and Central Europe, Africa, Asia and Latin America. Those present called for measures to alert medical professionals world-wide to the hazards of the use of DES, and requested measures to effect an international ban of DES in medication for pregnant women.
In the late 1990s our organisation DES Action Australia-NSW, upsettingly, received reports of current DES usage in pregnancy from overseas doctors who are employed or studying in Australia. In one scenario, our organisation was presenting a general talk to overseas doctors about the role and function of community support groups. Upon receiving information about the hazards of DES for the first time, a number of these doctors were understandably traumatised through their knowledge of the current practice of DES usage in pregnancy in their respective countries. The countries involved were Pakistan, Bangladesh and Sri Lanka.
So far, our organisation has found no evidence of any actions addressing the very serious concerns raised at the 1992 Swedish symposium.
INTERNATIONAL DES ACTION CONTACTS
DES Action USA (International) http://www.desaction.org/
DES Action Canada www.web.net/~desact
Reseau France reseaudesfrance@wanadoo.fr
DES Action Ireland http://www.desaction.ie/
DES Centrum, The Netherlands http://www.descentrum.nl/
Tuesday, 7 April 2009
NEW BOOK FOR YOUR READING LIST!

Science journalist, Susan Cohen and Christine Cosgrove, a WebMD contributor, both from USA, offer their riveting book detailing attempts to manipulate the height of children and the ethics of these experimental treatments. Doctors, drug companies and others who should know, freely admit there is no way, except in rare instances, of predicting anyone’s height at maturity. The motives of all the key players - parents, doctors and drug companies are questioned. The stories of the boys and girls themselves are told, many of them now grown and who were subjected to a wide range of non-FDA approved medical procedures. These consisted of extremes doses of oestrogen, pituitary glands taken from both animals and human cadavers, and testosterone injections, often with disastrous side effects.
In USA, it is estimated that thousands of tall girls were prescribed DES – often 100 times the amount of oestrogen delivered in a high dose birth control pill – daily over a period of years. These doses sent them prematurely into puberty and forced their growth plates to close. There are treated girls now in their 30s, 40s, 50s and 60s who blame these huge oestrogen doses for their myriad of health problems – including weight gain, ovarian cysts, miscarriages, blood clots, endometriosis, depression and infertility. In the early 1970s doctors switched tall girls over to different types of oestrogen when DES was found to cause cancer in the offspring of women prescribed it during pregnancy.
The book includes the plight and eventual success of the Australian group, Tall Girls Inc (chaired by Janet Cregan-Wood) in their demand for a DES follow-up study. The Australian researchers, who tracked down several hundred women and their parents, discovered that some families were still unable to talk about the subject. One woman said her teenage daughter had run away years earlier rather than take the pills, and she beseeched the researchers to let her know if they found her.
Cohen and Cosgrove pull no punches in this book. They note there have been many more incentives to encourage treatment with hormones than to study the drugs’ long-term effects.
Saturday, 28 February 2009
Safety of being 'in the know'

To keep up to date with vital information about DES exposure and at the same time support the great work of DES Action Australia-NSW, you may wish to subscribe annually to receive The DESfactor newsletter – only $20 (3 issues per year).
What’s in it for you?
By subscribing, it is a kind of insurance policy so that you can receive news developments in DES exposure health care that directly affect you and your family and - it’s hot off the press! You will also get news of what is brewing in the continuing research in DES exposure. You will be able to view firsthand the lobbying directions of the DES Action movement internationally and here in Australia and have the opportunity to be involved.
What more could you want?
Subscribe by sending your details (name, address, phone and email) along with cheque/money order to ‘DES Support NSW’ and send to: DES Action Australia-NSW, 14 Edmundson Cl, Thornleigh NSW 2120.
You can also conveniently make a deposit for subscription in DES Action Australia-NSW account: Westpac BSB: 032-087 Account: 11-9974 Account name: DES Support NSW. Notify us by email with your details (and deposit details) if you have done this.
All donations are gratefully accepted!
Please note: DES Action Australia-NSW operates separately and independently of the Melbourne-based group, DES Action Australia.