4th November 2008
Anti-miscarriage drug’s dire side effects taken to court
An Australian woman has won a 3 year legal battle in the United States, after taking legal recourse against the makers of anti-miscarriage drug DES (diethylstilbestrol).
Exposed to DES in-utero in the 1960’s, she developed the aggressive form of cancer clear cell adenocarcinoma at 21 years old, and was left infertile after a radical hysterectomy.
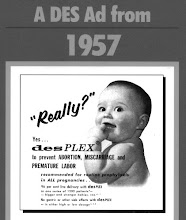
The claimant is not alone in her suffering of the clear cell cancer as a result of DES. In Australia, the Therapeutic Goods Administration (TGA) has documented a further case of this cancer in a 4 year old, diagnosed in 1957, and who subsequently died at age 16. A total 17 cancer cases have been recorded in Australia. The cancer incidence is about 1:1000 women exposed in-utero.
TGA data shows there are 68,000 DES exposed Australians - women prescribed DES in pregnancy and offspring exposed in-utero, but with further investigation of data held by the Australian Institute of Health and Welfare, the figure could be as high as 156,000.
The ability for DES exposed women to take legal action in the United States is a major
advance as the drug was not under patent in Australia. Numerous legal firms believe there is no possibility of legal action in Australia, leaving women with no recourse for their injuries.
Attorney Sybil Shainwald, a pioneer of landmark law in of New York City for DES victims, represented these women and says “Australian women who have been exposed to DES, and are infertile, have problems with their reproductive tract, have had cancer, or have been physically damaged in other ways that they believe are related to their exposure to the drug, may have the option of pursuing legal claims in courts of the United States.”
DES was given to pregnant women for the prevention of miscarriage, and injuries to their offspring did not appear until years after the initial exposure to the drug, in-utero. DES was taken off the U.S. and Australian market in 1971, but continued to circulate in the world marketplace for several years thereafter.
Coordinator of the organisation DES Action NSW, Carol Devine, says their members are heartened by the recent legal development. Their organisation is lobbying Federal Government for the promotion of DES information directly to women attending BreastScreen and Cervical Screening Programs to help in the early detection of DES associated cancer.
-ENDS-
For more information, contact:
Carol Devine, DES Action NSW (02) 98754820.
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment