 www.businessweek.com/print/lifestyle/content/healthday/649631.html
www.businessweek.com/print/lifestyle/content/healthday/649631.html Over the past few years there have been articles stating that doctors have been prescribing progesterone to pregnant women, based only on anecdotal reports of its effectiveness in preventing miscarriage. The US Food & Drug Administration (FDA) has now approved Makena (hydroxyprogesterone caproate) to prevent premature births.
For its approval, the FDA only reviewed a trial of 463 pregnant women and reviewed the development their babies only between 2.5 to 5 years of age. This drug is a reissue of an earlier drug and received accelerated approval. Makena's ingredient, hydroxyprogesterone caproate, has had previous safety concerns, including possible association with second trimester miscarriages. For more information about this see http://en.wikipedia.org/wki/17-Hydroxyprogesterone_caproate
TV Press article (www.presstv/ir/detail/163935.html) states: Further studies, however, are needed to show whether the infants whose births were delayed by the use of this drug will suffer fewer health and developmental problems or not.
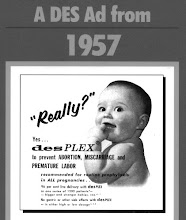
For all those familiar with the DES story, this approval by the US FDA rings alarm bells.
3 comments:
Thanks, Carol. Apparently the FDA learned NOTHING from the DES disaster. Appreciate your post!
US Senators John Kerry and Scott Brown are working on an apology from the US FDA re: the DES drug disaster. In light of Makena's accelerated approval, the FDA needs to be reminded of what happens with hormones and pregnancy -- the FDA hasn't learned its lesson! Please write or call the offices of Kerry and Brown, to ask them to keep championing the DES apology from the FDA. The following link contains contact info and a possible message for the US Senators: http://www.facebook.com/note.php?saved&¬e_id=191501700869312&id=233795428655#!/notes/wonder-drug/keep-pushing-the-fda-for-an-apology/191501700869312
From wikipedia "17α-Hydroxyprogesterone caproate is a synthetic steroid hormone that is similar to medroxyprogesterone acetate...".
Medroxyprogesterone acetate (Depo Provera) is the drug that's usually used to chemically castrate sex offenders in the U.S., and yet here are the FDA approving a drug with similar properties for use in pregnant women (at what look like extremely high doses too).
Has no one thought about what effect this might have on a male fetus? The way I see it, there's certainly the potential for the treatment to shut down testosterone production in a male fetus, which would have catastrophic consequences because brain masculinisation is driven by the action of testosterone.
The brain starts undergoing its sexually dimorphic development from around 16 weeks after conception, which so happens to be when treatment with Makena (or its generic equivalent) is often commenced. It's difficult to imagine a scenario better suited to creating a baby with a male body but a female brain.
If you've been wondering why there's so many stories about men wanting to become women in the news lately, there's your answer!
Post a Comment