 The following is copied from news released yesterday and it could well be a stepping stone for our long awaited apology for the DES tragedy from the US FDA:
The following is copied from news released yesterday and it could well be a stepping stone for our long awaited apology for the DES tragedy from the US FDA:(www.scottbrown.senate.gov/public/index.cfm/2011/2/kerry-brown-fda-finally-recognizes-prescription-drug-tragedy)
Feb 24 2011
Kerry, Brown:FDA Finally Recognizes Prescription Drug Tragedy
BOSTON - Forty years after doctors at Massachusetts General Hospital first discovered a link between the drug Diethylstilbestrol (DES) and a rare form of cancer, the FDA has sent a letter to Senators John Kerry and Scott Brown acknowledging that the widespread prescription of DES was a tragedy with devastating health consequences. The letter also outlines FDA's new initiatives to prevent similar drug disasters in the future. This letter comes in response to a letter the Senators sent to the FDA Commisioner Margaret Ann Hamburg last October on behalf of constituents affected by DES.
"The FDA is the first of defense for patients nationwide who need to know the medicines they use are safe, and it's important that today they've recognized the heartbreaking consequences that DES had for millions of people over the thirty years it was deemed safe."
"The FDA's acknowledgement of the devastating impact DES has had on patients across the country is long overdue, and I am pleased to hear of their admission, "said Sen. Brown.
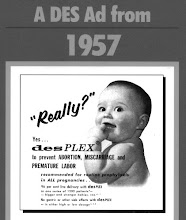
Diethylstilbestrol (DES) was a synthetic estrogen that was developed to supplement a woman's natural estrogen production. Originally prescribed by physicians in 1938 for women who experienced miscarriages or premature deliveries, DES was considered safe and effective for both mother and developing baby until the New England Journal of Medicine published a report in 1971 suggesting a link between DES use by pregnant women and a rare vaginal cancer in female offspring.
The Food and Drug Administration (FDA) subsequently issued a Drug Bulletin to physicians advising them to discontinue prescribing DES in pregnant women. According to the Centers for Disease control (CDC), approximately 5-10 million persons were exposed to DES between 1938-1971, including pregnant women and the children born from those pregnancies.
More news: The Boston Globe in a following article, mentions the request for an apology with "DES and birth defects; FDA acknowledges 'tragedy'from 40 years ago". Find this at http://www.boston.com/lifestyle/health/blog/dailydose/2011/02/des_and_birth_d.html
Feel free to register to make comments on this Boston Globe article!
1 comment:
The letter outlines FDA's new initiatives to prevent similar drug disasters in the future. The FDA is the first of defense for patients nationwide who need to know the medicines they use are safe.
Post a Comment